To be considered tamper resistant.
Prescription pad security requirements.
There are already a number of security measures that have been built into prescription forms to prevent theft and fraudulent use such as ultraviolet markings coloured backgrounds and serial numbers.
Reporting requirements for lost or stolen prescription pads.
Organisations holding stocks of prescription forms are responsible for their management and use by.
If a prescription is scanned or.
All controlled substance prescriptions written by indiana licensed practitioners as defined by ic 16 42 19 5 must comply with dea requirements as well as with 856 iac 1 34 and ic 16 42 22 6 in order for their prescriptions to be accepted for filling in indiana licensed pharmacies indiana licensed practitioners must use controlled substance prescription blanks.
The doj approved list of security prescription printers is available here.
For additional information on new requirements effective january 1 2019 view the board s memorandum on ab 1753 low chapter 479.
Who handle prescription forms are vigilant in adhering to procedures that reduce the risk of prescription theft and misuse.
Prescription pads under the medicaid program stating that payment shall not be made for.
Modification of information written on the prescription pad by the prescriber.
Helps providers develop local systems to ensure the security of prescription forms against theft and misuse.
Amounts expended for medical assistance for covered outpatient drugs for which the prescription was executed in written and non electronic form unless the prescription was executed on a tamper resistant pad 10.
Approved list of security prescription printers.
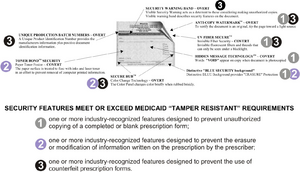
One or more industry recognized features designed to prevent the use of counterfeit prescription forms.
Features that would make the prescription tamper resistant include certain ty pes of paper as well as certain items that can be pre printed on the paper.
1 a latent repetitive void pattern shall be printed across the entire front of the prescription blank.
By october 1 2008 a prescription pad must contain all three of the above characteristics.
Tamper resistant pad as a result features added to the prescription after they are printed do not meet the requirement of the statute.
Preventing theft and misuse through secure storage.
Beginning january 1 2019 health and safety code section 11162 1 a 15 will require prescription forms for controlled substances to be printed with a uniquely serialized number.
Applies to blank computer prescription forms and handwritten pads.